Lesson Objectives
- Define elements and compounds.
- Explain why carbon is essential to life on Earth.
- Describe the structure and function of the four major types of organic compounds.
WORKBOOK ASSIGNMENT
Chapter 2.1 workbook pages
Get the workbook here: https://guesthollow.com/store/free-high-school-biology-workbook/
Vocabulary
- amino acid
- small molecule that is a building block of proteins
- carbohydrate
- organic compound such as sugar or starch
- chemical bond
- force that holds molecules together
- chemical reaction
- process that changes some chemical substances into others
- complementary base pair
- pair of nucleotide bases that bond together—either adenine and thymine (or uracil) or cytosine and guanine
- compound
- substance with a unique, fixed composition that consists of two or more elements
- DNA
- double-stranded nucleic acid that makes up genes and chromosomes
- double helix
- double spiral shape of the DNA molecule
- element
- pure substance that cannot be broken down into other types of substances
- lipid
- organic compound such as fat or oil
- matter
- anything that takes up space and has mass
- monosaccharide
- simple sugar such as glucose that is a building block of carbohydrates
- nucleic acid
- organic compound such as DNA or RNA
- nucleotide
- small molecule containing a sugar, phosphate group, and base that is a building block of nucleic acids
- organic compound
- compound found in living things that contains mainly carbon
- polynucleotide
- chain of nucleotides that alone or with another such chain makes up a nucleic acid
- polypeptide
- chain of amino acids that alone or with other such chains makes up a protein
- polysaccharide
- chain of monosaccharides that makes up a complex carbohydrate such as starch
- protein
- organic compound made up of amino acids
- RNA
- single-stranded nucleic acid that helps make proteins
- saturated fatty acid
- molecule in lipids in which carbon atoms are bonded to as many hydrogen atoms as possible
- unsaturated fatty acid
- molecule in lipids in which some carbon atoms are bonded to other groups of atoms rather than to hydrogen atoms
Introduction
If you look at your hand, what do you see? Um, your hand? NOT the answer I’m looking for. SKIN! There we go. You see skin, which consists of cells. But what are skin cells made of? Like all living cells, they are made of matter. In fact, all things are made of matter. Matter is anything that takes up space and has mass (the quantity of matter in an object). Matter, in turn, is made up of chemical substances. In this lesson you will learn about the chemical substances that make up living things.
Chemical Substances
A chemical substance is matter that has a definite composition. It also has the same composition throughout. A chemical substance may be either an element or a compound.
Elements
An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom. An atom is the smallest particle of an element that still has the properties of that element.
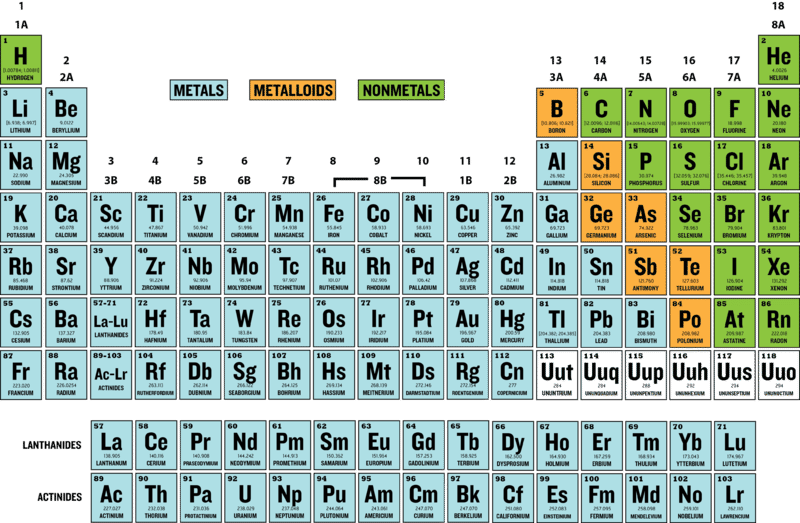
There are almost 120 known elements. As you can see from Figure below, the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals.
Compounds
A compound is a substance that consists of two or more elements. A compound has a unique composition that is always the same. The smallest particle of a compound is called a molecule. Consider water as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H2O. A model of a water molecule is shown in Figure below.

What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds. A chemical bond is a force that holds molecules together. Chemical bonds form when substances react with one another. A chemical reaction is a process that changes some chemical substances into others. A chemical reaction is needed to form a compound. Another chemical reaction is needed to separate the substances in a compound.
The Significance of Carbon
A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it could not exist. Why is carbon so basic to life? The reason is carbon’s ability to form stable bonds with many elements, including itself. This property allows carbon to form a huge variety of very large and complex molecules. In fact, there are nearly 10 million carbon-based compounds in living things! However, the millions of organic compounds can be grouped into just four major types: carbohydrates, lipids, proteins, and nucleic acids. You can compare the four types below. Each type is also described below.
| Type of Compound | Examples | Elements | Functions |
|---|---|---|---|
| Carbohydrates | sugars, starches | carbon, hydrogen, oxygen | provides energy to cells, stores energy, forms body structures |
| Lipids | fats, oils | carbon, hydrogen, oxygen | stores energy, forms cell membranes, carries messages |
| Proteins | enzymes, antibodies | carbon, hydrogen, oxygen, nitrogen, sulfur | helps cells keep their shape, makes up muscles, speeds up chemical reactions, carries messages and materials |
| Nucleic Acids | DNA, RNA | carbon, hydrogen, oxygen, nitrogen, phosphorus | contains instructions for proteins, passes instructions from parents to offspring, helps make proteins |
Energy From Carbon?
It may look like waste, but to some people, it’s green power.
Carbohydrates
Note: There is a brief mention of evolution towards the end (why humans love sugar).
Carbohydrates by Bozeman Science:
TEDEd: How do Carbohydrates impact your health?
Carbohydrates
Carbohydrates are the most common type of organic compound. A carbohydrate is an organic compound such as sugar or starch, and is used to store energy. Like most organic compounds, carbohydrates are built of small, repeating units that form bonds with each other to make a larger molecule. In the case of carbohydrates, the small repeating units are called monosaccharides.
Monosaccharides
A monosaccharide (mon-oe-sack-uh-ride) is a simple sugar such as fructose or glucose. Fructose is found in fruits, whereas glucose generally results from the digestion of other carbohydrates. Glucose is used for energy by the cells of most organisms.
Polysaccharides
A polysaccharide is a complex carbohydrate that forms when simple sugars bind together in a chain. Polysaccharides may contain just a few simple sugars or thousands of them. Complex carbohydrates have two main functions: storing energy and forming structures of living things. Some examples of complex carbohydrates and their functions are shown in Table below. Which type of complex carbohydrate does your own body use to store energy?
Complex Carbohydrates
| Name | Function | Example |
|---|---|---|
| Starch | Used by plants to store energy. |
A potato stores starch in underground tubers.
|
| Glycogen | Used by animals to store energy. |
A human being stores glycogen in liver cells.
|
| Cellulose | Used by plants to form rigid walls around cells. |
Plants use cellulose for their cell walls.
|
| Chitin | Used by some animals to form an external skeleton. I wonder if the tops of Klingon heads are formed from chitin. 😉 |
A housefly uses chitin for its exoskeleton.
|
KQED: Biofuels: From Sugar to Energy
For years there’s been buzz – both positive and negative – about generating ethanol fuel from corn. But thanks to recent developments, the Bay Area of California is rapidly becoming a world center for the next generation of green fuel alternatives. The Joint BioEnergy Institute is developing methods to isolate biofeuls from the sugars in cellulose. Watch the video Biofuels: Beyond Ethanol below for further information. Note: This video says the scientists are using evolution by forcing mutations.
A lipid is an organic compound such as fat or oil. Organisms use lipids to store energy, but lipids have other important roles as well. Lipids consist of repeating units called fatty acids. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids.
Saturated Fatty Acids
In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. This causes the molecules to form straight chains, as shown in Figure below. The straight chains can be packed together very tightly, allowing them to store energy in a compact form. This explains why saturated fatty acids are solids at room temperature (like butter!). Animals use saturated fatty acids to store energy.
Don’t fatty acids look kind of like fat caterpillars? Just sayin.

Unsaturated Fatty Acids
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they are bonded to other groups of atoms. Wherever carbon binds with these other groups of atoms, it causes chains to bend (see Figure above). The bent chains cannot be packed together very tightly, so unsaturated fatty acids are liquids at room temperature (like olive oil). Plants use unsaturated fatty acids to store energy. Some examples are shown in Figure below.

Types of Lipids
Lipids may consist of fatty acids alone, or they may contain other molecules as well. For example, some lipids contain alcohol or phosphate groups. They include
- triglycerides: (tri-glis-er-rides) the main form of stored energy in animals
- phospholipids: (foss-foe-lip-ids) the major components of cell membranes
- steroids: serve as chemical messengers and have other roles
Proteins
A protein is an organic compound made up of small molecules called amino acids. There are 20 different amino acids commonly found in the proteins of living things. Small proteins may contain just a few hundred amino acids, whereas large proteins may contain thousands of amino acids. Doesn’t that make me sound smart to use the word “whereas”? I thought I’d point that out, in case you didn’t notice.
By the way, the human body can synthesize all of the amino acids necessary to build proteins except for 10 called the “essential amino acids”. A human has to get those 10 essential amino acids from a healthy diet. If you fail to get even ONE of the 10 essential amino acids, there can be really serious health implications like muscle loss, fatigue, weakness, increased stress and anxiety levels, decreased immune response and changes in hair and skin texture.


Now, go make some food and tell your mom it’s for science.
Protein Structure
When amino acids bind together, they form a long chain called a polypeptide. A protein consists of one or more polypeptide chains. A protein may have up to four levels of structure. The lowest level, a protein’s primary structure, is its sequence of amino acids. Higher levels of protein structure are described in Figure below. The complex structures of different proteins give them unique properties, which they need to carry out their various jobs in living organisms. You can learn more about protein structure by watching the animation at the link below. http://www.stolaf.edu/people/giannini/flashanimat/proteins/protein%20structure.swf

Functions of Proteins
Proteins play many important roles in living things. Some proteins help cells keep their shape, and some make up muscle tissues. Many proteins speed up chemical reactions in cells. Other proteins are antibodies, which bind to foreign substances such as bacteria and target them for destruction. Still other proteins carry messages or materials. For example, human red blood cells contain a protein called hemoglobin, which binds with oxygen. Hemoglobin allows the blood to carry oxygen from the lungs to cells throughout the body. A model of the hemoglobin molecule is shown in Figure below.


A short video describing protein function can be viewed at http://www.youtube.com/watch?v=T500B5yTy58 (4:02).
Nucleic (new-clay-ic) Acids
Here’s a fun video that explains biomolecules:
Here’s a video that explains nucleic acids (there is a brief mention of evolution):
If it’s O.K. with your parent, you can try out a game where you learn about and design RNA molecules. The web address is:
Make sure your parent knows there is a chat box on the site. The chat box is not accessible to type in, unless you log in. You don’t need to log in to play the first several levels.
A nucleic acid is an organic compound, such as DNA or RNA, that is built of small units called nucleotides. Many nucleotides bind together to form a chain called apolynucleotide. The nucleic acid DNA (deoxyribonucleic acid) consists of two polynucleotide chains. The nucleic acid RNA (ribonucleic acid) consists of just one polynucleotide chain.
Structure of Nucleic Acids
Each nucleotide consists of three smaller molecules:
- sugar
- phosphate group
- nitrogen base
If you look at Figure below, you will see that the sugar of one nucleotide binds to the phosphate group of the next nucleotide. These two molecules alternate to form the backbone of the nucleotide chain. The nitrogen bases in a nucleic acid stick out from the backbone. There are four different types of bases: cytosine, adenine, guanine, and either thymine (in DNA) or uracil (in RNA). In DNA, bonds form between bases on the two nucleotide chains and hold the chains together. Each type of base binds with just one other type of base: cytosine always binds with guanine, and adenine always binds with thymine. These pairs of bases are called complementary base pairs.
Here’s a trick to help you remember which bases pair together: The Cool Guys stick together! (cytosine and guanine) That leaves adenine and thymine and their first letters spell the word AT.

The binding of complementary bases allows DNA molecules to take their well-known shape, called a double helix, which is shown in Figure below. A double helix is like a spiral staircase. The double helix shape forms naturally and is very strong, making the two polynucleotide chains difficult to break apart. The structure of DNA will be further discussed in the chapter Molecular Genetics: From DNA to Proteins.

An animation of DNA structure can be viewed at http://www.youtube.com/watch?v=qy8dk5iS1f0.
Roles of Nucleic Acids
DNA is found in genes, and its sequence of bases makes up a code. Between “starts” and “stops,” the code carries instructions for the correct sequence of amino acids in a protein (see Figure above). RNA uses the information in DNA to assemble the correct amino acids and help make the protein. The information in DNA is passed from parent cells to daughter cells whenever cells divide. The information in DNA is also passed from parents to offspring when organisms reproduce. This is how inherited characteristics are passed from one generation to the next.
Lesson Summary
- Living things consist of matter, which can be an element or a compound. A compound consists of two or more elements and forms as a result of a chemical reaction.
- Carbon’s unique ability to form chemical bonds allows it to form millions of different large, organic compounds. These compounds make up living things and carry out life processes.
- Carbohydrates are organic compounds such as sugars and starches. They provide energy and form structures such as cell walls.
- Lipids are organic compounds such as fats and oils. They store energy and help form cell membranes in addition to having other functions in organisms.
- Proteins are organic compounds made up of amino acids. They form muscles, speed up chemical reactions, and perform many other cellular functions.
- Nucleic acids are organic compounds that include DNA and RNA. DNA contains genetic instructions for proteins, and RNA helps assemble the proteins.
Lesson Review Questions
Recall
1. What are elements and compounds? Give an example of each.
2. List the four major types of organic compounds.
3. What determines the primary structure of a protein?
4. State two functions of proteins.
5. Identify the three parts of a nucleotide.
Apply Concepts
6. Butter is a fat that is a solid at room temperature. What type of fatty acids does butter contain? How do you know?
7. Assume that you are trying to identify an unknown organic molecule. It contains only carbon, hydrogen, and oxygen and is found in the cell walls of a newly discovered plant species. What type of organic compound is it?
Think Critically
8. Explain why carbon is essential to all known life on Earth.
9. Compare and contrast the structures and functions of simple sugars and complex carbohydrates.
10. Explain why molecules of saturated and unsaturated fatty acids have different shapes.
Points to Consider
Large organic compounds consist of many smaller units that are linked together in chains.
- How can the smaller units become linked together? What process do you think is involved?
- What do you think holds the smaller units together in a chain?
Previous: Biology the Study of Life
Next: Biochemical Reactions

CK-12 Foundation is licensed under Creative Commons AttributionNonCommercial 3.0 Unported (CC BY-NC 3.0)”
• Terms of Use • Attribution








Im looking over this and im overwhelmed. I think its great I just dont know how i would use this everyday as it is online??? Do I come to this website each day and click on the chapter and just read with him?? Or do I orint it all out and write the video websites down?? I want to do this but Im confused on how to use it?
Hello! I made a weekly/daily schedule that helps students know what they are to do each day:
https://guesthollow.com/guest-hollows-high-school-biology-curriculum-online-schedule/
For example, if you look at week one:
https://guesthollow.com/guest-hollows-high-school-biology-curriculum-online-schedule/biology-week-1/
You can see the days specific chapters are supposed to be read. A student can click directly on the link in the schedule to go to the online textbook by himself. Because the schedule has things you can pick-and-choose from, you can tell your student which of the weekly assignments he can ignore. If you have the printable version of the schedule for him to reference, you can just cross things out that you don’t plan on doing (specific labs, etc.).
So, an example day (day 1 of starting the program). You’d have the schedule landing page bookmarked. Your son would go to the page, click on week 1. Then he would look at the column for day 1 and see what he is supposed to do. He’d click on the link for chapter 1.1 and read through about half of it. He’d work on answering some of the workbook pages as he reads through the online text. Then if you were doing the IGHBE labs (I recommend you look through the lab planner page to decide which labs you want to do for the year ahead of time): https://guesthollow.com/biology-lab-and-activities-supply-list/
Anyway, if you planned to do that particular lab. The next thing would be to read chapter 1 of the Answers book (if you wanted to use that book). Then there would be a couple optional videos and a controls and variables worksheet. That’s it for day 1. That’s how using the program would work. Let me know if you have other questions! 🙂
The links in your reply send me to pages that say “Oops! That page can’t be found.”
Hello! Our website was totally overhauled. I just edited that old reply with the new links. 🙂 Thank you for the heads-up, so I could fix that!
Also, here is a link that explains how to use most of our curriculums:
https://guesthollow.com/how-to-use-and-print-guest-hollow-materials/